- Atomic model
- Basic properties
- The electron
- The nucleus
- Development of atomic theory
- The beginnings of modern atomic theory
- Studies of the properties of atoms
- Models of atomic structure
- Advances in nuclear and subatomic physics
Atom Learning automates and surpasses the capabilities of even the most proficient tutor in a way that's fun and engaging for pupils. WCCS is thrilled to support their good work and long may it continue! Neil McLaughlan, Headmaster Westminster Cathedral Choir School. On the wall miter station. Just how small are atoms? And what's inside them? The answers turn out to be astounding, even for those who think they know. This fast-paced animation uses s.
Atom Longboards. Established in 2005, by ridersfor riders, we make a wide range of affordable longboard styles, all while refusing to sacrifice quality. More than ten years down the road, Atom is running stronger than ever featuring drop deck, drop through, freeride, cruiser, downhill, all-terrain. Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element. As such, the atom is the basic building block of chemistry.
Our editors will review what you’ve submitted and determine whether to revise the article.
Join Britannica's Publishing Partner Program and our community of experts to gain a global audience for your work!Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element. As such, the atom is the basic building block of chemistry.
Most of the atom is empty space. The rest consists of a positively charged nucleus of protons and neutrons surrounded by a cloud of negatively charged electrons. The nucleus is small and dense compared with the electrons, which are the lightest charged particles in nature. Electrons are attracted to any positive charge by their electric force; in an atom, electric forces bind the electrons to the nucleus.
Because of the nature of quantum mechanics, no single image has been entirely satisfactory at visualizing the atom’s various characteristics, which thus forces physicists to use complementary pictures of the atom to explain different properties. In some respects, the electrons in an atom behave like particles orbiting the nucleus. In others, the electrons behave like waves frozen in position around the nucleus. Such wave patterns, called orbitals, describe the distribution of individual electrons. The behaviour of an atom is strongly influenced by these orbital properties, and its chemical properties are determined by orbital groupings known as shells.
This article opens with a broad overview of the fundamental properties of the atom and its constituent particles and forces. Following this overview is a historical survey of the most influential concepts about the atom that have been formulated through the centuries. For additional information pertaining to nuclear structure and elementary particles, seesubatomic particles.
Atomic model
Most matter consists of an agglomeration of molecules, which can be separated relatively easily. Molecules, in turn, are composed of atoms joined by chemical bonds that are more difficult to break. Each individual atom consists of smaller particles—namely, electrons and nuclei. These particles are electrically charged, and the electric forces on the charge are responsible for holding the atom together. Attempts to separate these smaller constituent particles require ever-increasing amounts of energy and result in the creation of new subatomic particles, many of which are charged.
As noted in the introduction to this article, an atom consists largely of empty space. The nucleus is the positively charged centre of an atom and contains most of its mass. It is composed of protons, which have a positive charge, and neutrons, which have no charge. Protons, neutrons, and the electrons surrounding them are long-lived particles present in all ordinary, naturally occurring atoms. Other subatomic particles may be found in association with these three types of particles. They can be created only with the addition of enormous amounts of energy, however, and are very short-lived.
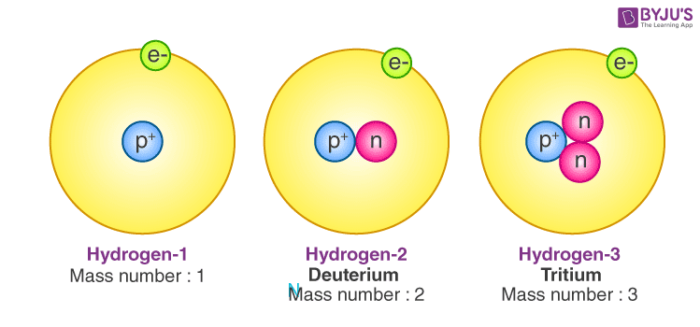
All atoms are roughly the same size, whether they have 3 or 90 electrons. Approximately 50 million atoms of solid matter lined up in a row would measure 1 cm (0.4 inch). A convenient unit of length for measuring atomic sizes is the angstrom (Å), defined as 10−10 metre. The radius of an atom measures 1–2 Å. Compared with the overall size of the atom, the nucleus is even more minute. It is in the same proportion to the atom as a marble is to a football field. In volume the nucleus takes up only 10−14 metres of the space in the atom—i.e., 1 part in 100,000. A convenient unit of length for measuring nuclear sizes is the femtometre (fm), which equals 10−15 metre. The diameter of a nucleus depends on the number of particles it contains and ranges from about 4 fm for a light nucleus such as carbon to 15 fm for a heavy nucleus such as lead. In spite of the small size of the nucleus, virtually all the mass of the atom is concentrated there. The protons are massive, positively charged particles, whereas the neutrons have no charge and are slightly more massive than the protons. The fact that nuclei can have anywhere from 1 to nearly 300 protons and neutrons accounts for their wide variation in mass. The lightest nucleus, that of hydrogen, is 1,836 times more massive than an electron, while heavy nuclei are nearly 500,000 times more massive.
Basic properties
/GettyImages-1143649584-04e88f5111ab4417bc8d8e3fe54566ef.jpg)

Be Atomic Mass
Atomic number
The single most important characteristic of an atom is its atomic number (usually denoted by the letter Z), which is defined as the number of units of positive charge (protons) in the nucleus. For example, if an atom has a Z of 6, it is carbon, while a Z of 92 corresponds to uranium. A neutral atom has an equal number of protons and electrons so that the positive and negative charges exactly balance. Since it is the electrons that determine how one atom interacts with another, in the end it is the number of protons in the nucleus that determines the chemical properties of an atom.
- key people
- related topics
Teletype for Atom
Great things happen when developers work together—from teaching and sharing knowledge to building better software. Teletype for Atom makes collaborating on code just as easy as it is to code alone, right from your editor.
Share your workspace and edit code together in real time. To start collaborating, open Teletype in Atom and install the package.
GitHub for Atom
A text editor is at the core of a developer’s toolbox, but it doesn't usually work alone. Work with Git and GitHub directly from Atom with the GitHub package.
Create new branches, stage and commit, push and pull, resolve merge conflicts, view pull requests and more—all from within your editor. The GitHub package is already bundled with Atom, so you're ready to go!
Everything you would expect
Cross-platform editing
Atom works across operating systems. Use it on OS X, Windows, or Linux.
Built-in package manager
Search for and install new packages or create your own right from Atom.
Smart autocompletion
Lagu hijau daun suara ku berharap mp3. Atom helps you write code faster with a smart and flexible autocomplete.
File system browser
Be Atomic Weight
Easily browse and open a single file, a whole project, or multiple projects in one window.
Multiple panes
Split your Atom interface into multiple panes to compare and edit code across files.
Find and replace
Find, preview, and replace text as you type in a file or across all your projects.
Make it your editor
Packages
Flv crunch for windows. Choose from thousands of open source packages that add new features and functionality to Atom, or build a package from scratch and publish it for everyone else to use.
Themes
Atom comes pre-installed with four UI and eight syntax themes in both dark and light colors. Can't find what you're looking for? Install themes created by the Atom community or create your own.
Customization
It's easy to customize and style Atom. Tweak the look and feel of your UI with CSS/Less, and add major features with HTML and JavaScript.
See how to set up Atom
Under the hood
Be Atomic Radius
Atom is a desktop application built with HTML, JavaScript, CSS, and Node.js integration. It runs on Electron, a framework for building cross platform apps using web technologies.
Open source
Atom is open source. Be part of the Atom community or help improve your favorite text editor.

Keep in touch
| GitHub | github.com/atom |
| @AtomEditor | |
| Chat | Slack |
| Forum | Discuss |
| Stuff | Atom Gear |
| RSS Feed | Packages & Themes |
