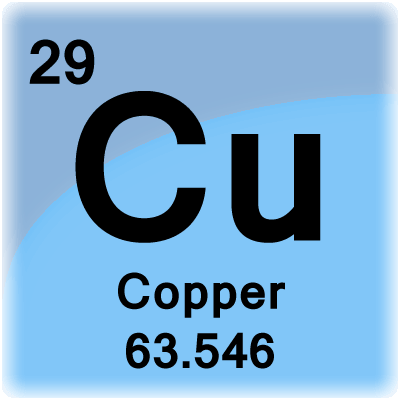
Copper is an Element, hence it does not have any formula but, a symbol i.e. When it forms a compound with any other element/radical, we can refer it from any formula like (Copper Sulphate is a compound having formula CuSO4). Bottomline: Elements have symbols, while Compounds have formulae. 29 Cu Copper 63.546. Atomic Number: 29. Atomic Weight: 63.546. Melting Point: 1357.77 K (1084.62°C or 1984.32°F) Boiling Point: 2835 K (2562°C or 4644°F) Density: 8.933 grams per cubic centimeter. Phase at Room Temperature: Solid. Element Classification: Metal. Period Number: 4. Group Number: 11. Group Name: none. What's in a name?
The Element Copper
[Click for Isotope Data]
Atomic Number: 29
Atomic Weight: 63.546
Melting Point: 1357.77 K (1084.62°C or 1984.32°F)
Boiling Point: 2835 K (2562°C or 4644°F)

Density: 8.933 grams per cubic centimeter
Phase at Room Temperature: Solid
Element Classification: Metal
Period Number: 4
Group Number: 11
Group Name: none
What's in a name? From the Latin word cuprum, which means 'from the island of Cyprus.'
Say what? Copper is pronounced as KOP-er.
History and Uses:
Archaeological evidence suggests that people have been using copper for at least 11,000 years. Relatively easy to mine and refine, people discovered methods for extracting copper from its ores at least 7,000 years ago. The Roman Empire obtained most of its copper from the island of Cyprus, which is where copper's name originated. Today, copper is primarily obtained from the ores cuprite (CuO2), tenorite (CuO), malachite (CuO3·Cu(OH)2), chalcocite (Cu2S), covellite (CuS) and bornite (Cu6FeS4). Large deposits of copper ore are located in the United States, Chile, Zambia, Zaire, Peru and Canada.
Used in large amounts by the electrical industry in the form of wire, copper is second only to silver in electrical conductance. Since it resists corrosion from the air, moisture and seawater, copper has been widely used in coins. Although once made nearly entirely from copper, American pennies are now made from zinc that has been coated with copper. Copper is also used to make water pipes and jewelry, as well as other items.
Pure copper is usually too soft for most uses. People first learned about 5,000 years ago that copper can be strengthened if it is mixed with other metals. The two most familiar alloys of copper are bronze and brass. Bronze, the first alloy created by people, is a mix of copper that contains as much as 25% tin. Early people used bronze to make tools, weaponry, containers and ornamental items. Brass, a mix of copper that contains between 5% and 45% zinc, was first used about 2,500 years ago. The Romans were the first to make extensive use of brass, using it to make such things as coins, kettles and ornamental objects. Today, brass is also used in some musical instruments, screws and other hardware that must resist corrosion.
Hydrated copper sulfate (CuSO4·H2O), also known as blue vitriol, is the best known copper compound. It is used as an agricultural poison, as an algicide in water purification and as a blue pigment for inks. Cuperic chloride (CuCl2), another copper compound, is used to fix dyes to fabrics. Cuprous chloride (CuCl) is a poisonous white powder that is chiefly used to absorb carbon dioxide (CO2). Copper cyanide (CuCN) is commonly used in electroplating.
Estimated Crustal Abundance: 6.0×101 milligrams per kilogram
Estimated Oceanic Abundance: 2.5×10-4 milligrams per liter
Number of Stable Isotopes: 2 (View all isotope data)
Ionization Energy: 7.726 eV
Oxidation States: +2, +1
Electron Shell Configuration: | 1s2 |
2s2 2p6 | |
3s2 3p6 3d10 | |
4s1 |
For questions about this page, please contact Steve Gagnon.
Element Copper - Cu
Comprehensive data on the chemical element Copper is provided on this page; including scores of properties, element names in many languages, most known nuclides of Copper. Common chemical compounds are also provided for many elements. In addition technical terms are linked to their definitions and the menu contains links to related articles that are a great aid in one's studies.
Copper Menu
- Copper Page One
- Copper Page Two
- Copper Page Three
Overview of Copper
- Atomic Number: 29
- Group: 11
- Period: 4
- Series: Transition Metals
Copper's Name in Other Languages
- Latin: Cuprum
- Czech: Měd´
- Croatian: Bakar
- French: Cuivre
- German: Kupfer - e
- Italian: Rame
- Norwegian: Kobber
- Portuguese: Cobre
- Russian: Медь
- Spanish: Cobre
- Swedish: Koppar
Atomic Structure of Copper
- Atomic Radius: 1.57Å
- Atomic Volume: 7.1cm3/mol
- Covalent Radius: 1.17Å
- Cross Section (Thermal Neutron Capture)σa/barns: 3.78
- Crystal Structure: Cubic face centered
- Electron Configuration:
- 1s2 2s2p6 3s2p6d10 4s1
- Electrons per Energy Level: 2,8,18,1
- Shell Model
- Shell Model
- Ionic Radius: 0.73Å
- Filling Orbital: 3d10
- Number of Electrons (with no charge): 29
- Number of Neutrons (most common/stable nuclide): 35
- Number of Protons: 29
- Oxidation States:2,1
- Valence Electrons: 3d10 4s1
Chemical Properties of Copper
- Electrochemical Equivalent: 1.1855g/amp-hr
- Electron Work Function: 4.65eV
- Electronegativity: 1.9 (Pauling); 1.75 (Allrod Rochow)
- Heat of Fusion: 13.05kJ/mol
- Incompatibilities:
- Oxidizers, alkalis, sodium azide, acetylene
- Ionization Potential
- First: 7.726
- Second: 20.292
- Third: 36.83
- Valence Electron Potential (-eV): 34


Physical Properties of Copper

Cu Element Atomic Number
- Atomic Mass Average: 63.546
- Boiling Point: 2840K 2567°C 4653°F
- Coefficient of lineal thermal expansion/K-1: 16.5E-6
- Conductivity
- Electrical: 0.596 106/cm Ω
Thermal: 4.01 W/cmK
- Electrical: 0.596 106/cm Ω
- Density: 8.96g/cc @ 300K
- Description:
- Reddish orange transition metal.
- Elastic Modulus:
- Bulk: 137.8/GPa
- Rigidity: 48.3/GPa
- Youngs: 129.8/GPa
- Enthalpy of Atomization: 338.9 kJ/mole @ 25°C
- Enthalpy of Fusion: 13.01 kJ/mole
- Enthalpy of Vaporization: 304.6 kJ/mole
- Flammablity Class: Non-combustible solid (except as dust)
- Freezing Point:see melting point
- Hardness Scale
- Brinell: 874 MN m-2
- Mohs: 3
- Vickers: 369 MN m-2
- Heat of Vaporization: 300.3kJ/mol
- Melting Point: 1357.75K 1084.6°C 1984.3°F
- Molar Volume: 7.11 cm3/mole
- Optical Reflectivity: 90%
- Physical State (at 20°C & 1atm): Solid
- Specific Heat: 0.38J/gK
- Vapor Pressure = 0.0505Pa@1084.6°C
Regulatory / Health
- CAS Number
- 7440-50-8
- RTECS: GL5325000
- NFPA 704
- Health: 2
- Fire:
- Reactivity:
- Special Hazard:
- OSHAPermissible Exposure Limit (PEL)
- TWA: 1 mg/m3
- OSHA PEL Vacated 1989
- TWA: 1 mg/m3
- NIOSHRecommended Exposure Limit (REL)
- TWA: 1 mg/m3
- IDLH: 100 mg/m3
- Routes of Exposure: Inhalation; Ingestion; Skin and/or eye contact
- Target Organs: Eyes, skin, respiratory system, liver, kidneys (increase(d) risk with Wilson's disease)
- Levels In Humans:
Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances.- Blood/mg dm-3: 1.01
- Bone/p.p.m: 1-26
- Liver/p.p.m: 30
- Muscle/p.p.m: 10
- Daily Dietary Intake: 0.50-6 mg
- Total Mass In Avg. 70kg human: 72 mg
Who / Where / When / How
- Discoverer: Known to ancient civilization
- Discovery Location: Unknown
- Discovery Year: Unknown
- Name Origin:
- Latin: cyprium (island of Cyprus famed for its copper mines).
- Abundance of Copper:
- Earth's Crust/p.p.m.: 50
- Seawater/p.p.m.:
- Atlantic Suface: 0.00008
- Atlantic Deep: 0.00012
- Pacific Surface: 0.00008
- Pacific Deep: 0.00028
- Atmosphere/p.p.m.: N/A
- Sun (Relative to H=1E12): 1.15
- Sources of Copper:
- Pure copper occurs rarely in nature. Usually copper found in such minerals as azurite, malachite and bornite and in sulfides as in chalcopyrite (CuFeS2), coveline (CuS), chalcosine (Cu2S) or oxides like cuprite (Cu2O). Copper is obtained by smelting, leaching and by electrolysis. Annual world production is around 6,540,000 tons. Primary mining areas are in USA, Zaire, Zambia, Canada, Chile, Cyprus, Russia and Australia.
- Uses of Copper:
- Most often used as an electrical conductor. Its alloys are used in jewelry, bronze sculptures and for coins. The skin of the Statue of Liberty is made of copper.
- Additional Notes:
- Copper is a very interesting element. It is one of the transition elements that actually uses electrons from one of the inner orbitals in chemical reactions. In addition, it has more than one oxidation state. Like many of the transition elements, copper has a colored ion. Copper typically forms a bluish green solution. Copper (Cu) has two valences Cu I (cuprous) has one valence electron and Cu II (cupric) has two valence electrons. Copper was one of the earliest known metals, having reportedly been mined for over 5000 years. In nature it has two isotopes, 63 (69.09%), which has 29 electrons and protons and 34 neutrons, and 65 (30.91%), which has 29 electrons and protons and 36 neutrons. Brass and bronze are alloys of copper.
Copper Menu
- Copper Page One
- Copper Page Two
- Copper Page Three
References
A list of reference sources used to compile the data provided on our periodic table of elements can be found on the main periodic table page.
Related Resources
- Anatomy of the Atom
Answers many questions regarding the structure of atoms. - Molarity, Molality and Normality
Introduces stoichiometry and explains the differences between molarity, molality and normality. - Molar Mass Calculations and Javascript Calculator
Molar mass calculations are explained and there is a JavaScript calculator to aid calculations. - Chemical Database
This database focuses on the most common chemical compounds used in the home and industry.
Citing this page
If you need to cite this page, you can copy this text:
What Is Cu Atomic Number
Kenneth Barbalace. Periodic Table of Elements - Copper - Cu. EnvironmentalChemistry.com. 1995 - 2021. Accessed on-line: 4/24/2021
https://EnvironmentalChemistry.com/yogi/periodic/Cu.html
.
Linking to this page
If you would like to link to this page from your website, blog, etc., copy and paste this link code (in red) and modify it to suit your needs:
<a href='https://EnvironmentalChemistry.com/yogi/periodic/Cu.html'>echo Periodic Table of Elements: Copper - Cu (EnvironmentalChemistry.com)</a>- Comprehensive information for the element Copper - Cu is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions.
.
NOTICE: While linking to articles is encouraged, OUR ARTICLES MAY NOT BE COPIED TO OR REPUBLISHED ON ANOTHER WEBSITE UNDER ANY CIRCUMSTANCES.
Cu Charge
PLEASE, if you like an article we published simply link to it on our website do not republish it.
